Background
Metastatic brain cancer increases the risk of thrombosis and intracranial hemorrhage (ICH). Immune Checkpoint Inhibitors (ICI) have emerged as breakthrough therapies for the treatment of metastatic disease. Thrombosis has been increasingly reported as a potential adverse event associated to ICI treatment. There is conflicting evidence on the comparative risk of thrombosis between ICI and chemotherapy, and limited data on bleeding outcomes during ICI treatment. We aimed to compare the rate of thrombosis and ICH in patients with metastatic brain tumors treated with ICI versus chemotherapy.
Methods
A retrospective, single-center cohort study was performed at Beth Israel Deaconess Medical Center. Patients with metastatic brain tumors, treated from 2010 to 2020 with ICI or chemotherapy were included. Primary endpoints included development of thrombotic and ICH events within 12 months of starting treatment. Thrombotic events included venous thromboembolism (VTE), or arterial thrombosis confirmed by imaging. ICH events were classified by a neuro-oncologist blinded to patient information. Intracranial bleeding due to surgical interventions or radiation therapy were excluded. Primary exposure was treatment group, defined as ICI with or without chemotherapy compared to chemotherapy alone. Proportions were compared using Chi square or Fisher's exact tests. Log-binomial regression was used to estimate relative risk (RR) and 95% confidence interval (CI) associated with each additional point of the Khorana score (KS). Cox hazard models were used to calculate the hazard of thrombosis and ICH in each group, accounting for death as a competing risk. Models were weighted by the inverse probability of treatment, estimated using age, gender, cancer, KS, elevated creatinine, and prior exposure to anticoagulants and/or antiplatelets (AC/AP) as covariates; weighting (IPTW) to calculate the cumulative incidence (CumI) of thrombosis and ICH among treatment groups, and stratified by use of AC/AP at the start of treatment.
Results
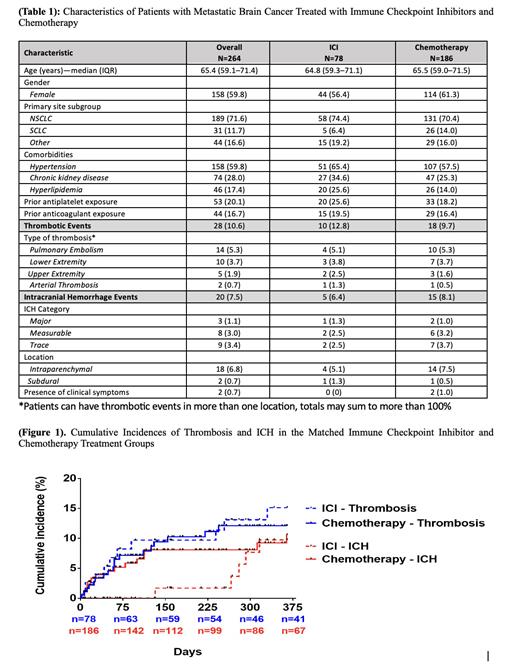
We included 264 patients with a median age of 65.4 years (Table 1). Overall, 59.8% were female. Major primary malignancies included non-small cell lung cancer (NSCLC) 189 (71.6%), SCLC 31 (11.7%), melanoma 13 (4.9%), and renal cell carcinoma 13 (2.9%). Regarding treatment, 78 (29.5%) patients received ICI, and 186 (70.5) chemotherapy; within the ICI group, 65.4% of patients also received chemotherapy. Overall, 28 patients developed thrombotic events, 14 pulmonary embolisms (50%), followed by lower extremity VTE (35.7%). Ten patients developed ICH during the study period, including three patients with major bleeds (>10cm3 in size and/or symptomatic). The majority of bleeding events were noted to be intraparenchymal (80%).
The 12-month weighted CumI of thrombosis was similar in the ICI (11.1%, 95%CI 4.5-17.3) and chemotherapy (9.2%, 95%CI 5.6-12.6) groups. The 12-month CumI of ICH was also similar between ICI (7.2%, 95%CI 1.7-12.4) and chemotherapy (8.9%, 95%CI 5.4-12.3) groups. (Figure 1) Median time (MT) to thrombosis was comparable for patients on ICI (58.5 days, IQR26.0-239) and chemotherapy (64.5 days, IQR 26.0-154) (P=1.00). Conversely, ICH occurred earlier in the chemotherapy group (MT 49.0 days, IQR 12-112) compared to the ICI group (MT 280 days, IQR 266-292) (P=0.01).
In weighted competing risk models, the hazard of thrombosis was similar among both treatment groups (aHR: 1.05, 95% CI: 0.5-2.1). The risk of ICH was not significantly lower with ICI compared to chemotherapy (aHR: 0.66, 95% CI 0.2-1.5).
Within the ICI group, AC/AP use was not associated with significant difference in thrombotic risk (aHR: 0.63, 95%CI 0.1-2.3) but there was a significantly increased risk of ICH (aHR: 9.0, 95%CI 1.0-79.3). Within the chemotherapy group, use of AC/AP was not associated with increased risk of thrombosis (aHR: 0.96, 95%CI 0.3-2.5) or ICH (aHR: 0.16, 95% CI 0.0-1.0).
Conclusions
Rates of thrombosis and ICH were high in patients with metastatic brain tumors treated with ICI or cytotoxic chemotherapy but did not differ significantly between the two treatment groups. In our cohort, the time to intracranial bleeding was significantly shorter among those on chemotherapy compared to those on ICI. Contrasting risks of ICH and thrombosis in patients with metastatic brain cancer should be considered during treatment.
Disclosures
Rangachari:AstraZeneca: Honoraria; Bristol Myers Squibb: Research Funding; Novocure: Research Funding; AbbVie/Stemcentrx: Research Funding; DynaMed: Honoraria; Advance Medica/TelaDoc: Honoraria; DAVA Oncology: Other: Travel support. Zwicker:Sanofi, CSL, Parexel: Consultancy; Pfizer/BMS, Portola, Daiichi: Honoraria; Incyte Corporation, Quercegen: Research Funding; Janssen: Consultancy; CSL Behring: Consultancy; Sanofi: Consultancy; calyx: Consultancy.